One-Step Electrodeposition of Iron Oxyhydroxide onto 3D Porous Graphene (Gii) Substrates for On Chip Asymmetric Micro-Supercapacitors
Highlights
- Electrodeposition of iron oxyhydroxide (FeOOH) onto carbon substrates based on acetate precursors was demonstrated. This Gii electrode outperformed the more established iron chloride deposition.
- Cycling stability of the fuel cell is reasonable at 70% retention at 5000 cycles.
Abstract
Electrochemical capacitors based on redox active materials can achieve greater capacitance values than traditional electric double layer composites.
Herein, electrodeposition of iron oxyhydroxide from a mildly acidic acetate precursor is reported. The one-step deposition resulted in a submicron film composed of FeOOH phase, which was confirmed via Raman and x-ray photoelectron spectroscopy.
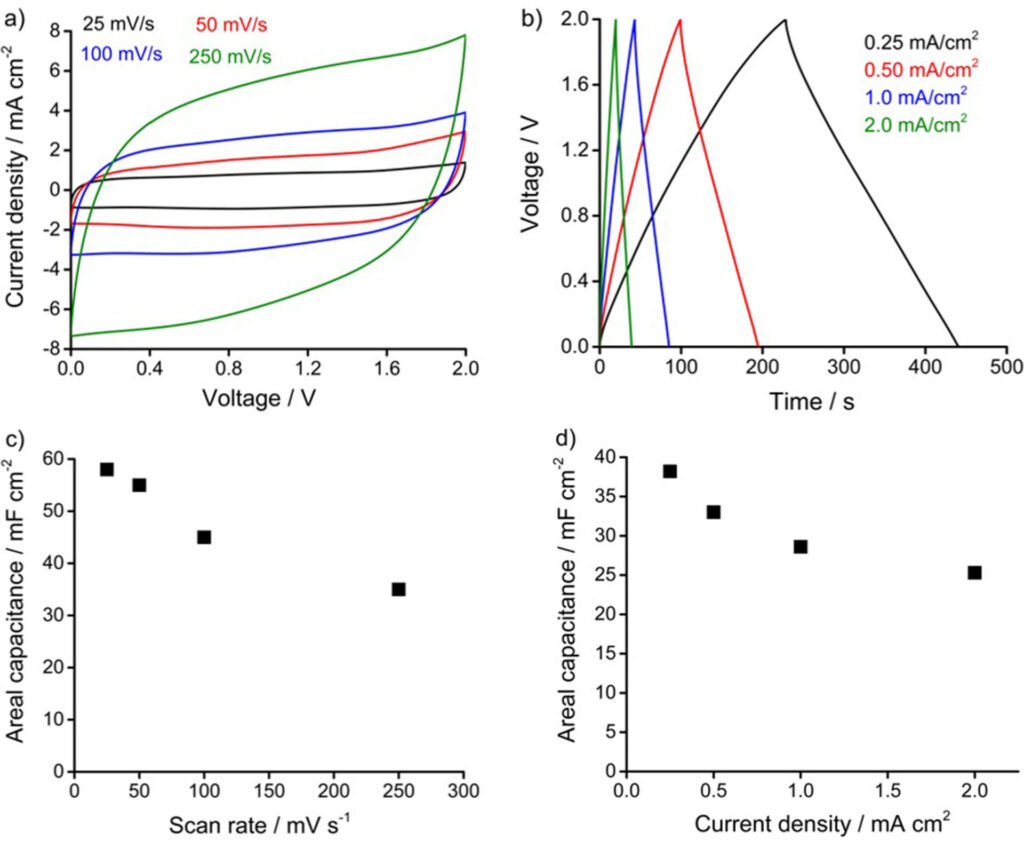
The capacitance increased linearly with loading amount and achieved a maximum at 1600 mC deposition with 120 mF cm-2 at 25 mV s-1 after which the film became more resistive, limiting electrolyte access to the porous graphene substrate.
The deposited FeOOH demonstrated promising rate capability and good cycling stability, without phase changes, retaining 82% of the initial capacitance after 5000 consecutive charge/discharge cycles.
The charge storage mechanism of FeOOH was determined via in situ Raman spectroscopy, which followed reversible iron oxygen vibration changes upon cycling which become more intense upon reduction as a result of sodium ion intercalation.
Furthermore, an asymmetric configuration full cell combining FeOOH/MnO2 allowed the working voltage to be extended to 2 V, maintaining an ideal capacitor behaviour, and achieving a maximum energy and power density of 21 uWh cm-2 and 2.5 mW cm-2 respectively
Introduction
Electrochemical capacitors are key components within electric mobility, portable electronics, and renewable energy supply applications.[1]
These devices are characterised by high-power density (fast-charging) and long cycle-life, requiring virtually no maintenance or replacement during application.[2]
On this account, electrochemical capacitors are being investigated for new applications in Internet-of-things (IoT) networks, miniaturised electronics, drug delivery and smart buildings, where autonomy is essential.[3-5]
State-of-the art devices in industry use a combination of high-surface area carbon electrodes and organic electrolytes, due to their large operation window.
Aqueous electrolytes have their commercial application hindered by the chemical stability of water (1.23 V) however, are an attractive alternative due to potentially lower cost, safety, sustainability and higher ionic mobility.[6] Polymer additives can form hydrogels which enable flexible on-chip microsupercapacitors.[7]


