Electrochemical Immunosensor Using COOH-Functionalized 3D Graphene Electrodes for Sensitive Detection of Tau-441 Protein
Highlights
- Development of a promising antibody-based Gii-Sens biosensor for the sensitive and selective detection of Tau-441 protein, an Alzheimer’s Disease biomarker.
- Detection levels down to the femtomolar (0.14 fM) and linear range of 1 fM to 10 nM.
- The simple, ultrasensitive electrochemical immunosensor presented in the publications show great potential in paving the way for the development of cost-effective diagnostics tools with broad applications in clinical research.
Abstract
Early diagnosis of Alzheimer’s disease (AD) is essential for effective treatment; however, current diagnostic methods are often complex, costly, and unsuitable for point-of-care testing.
Graphene-based biosensors offer an alternative due to their affordability, versatility, and high conductivity. However, graphene’s conductivity can be compromised when its carbon lattice is oxidised to introduce functional groups for biomolecule immobilisation.
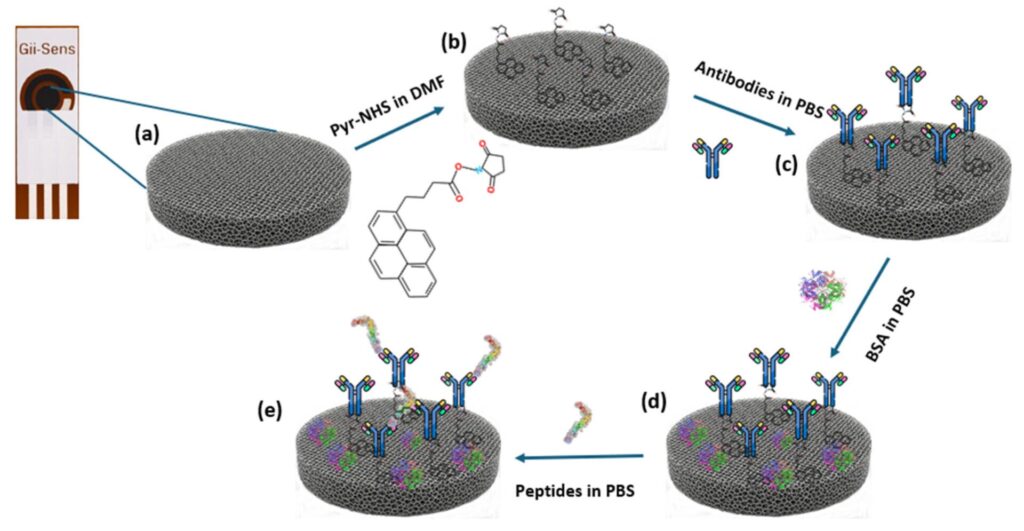
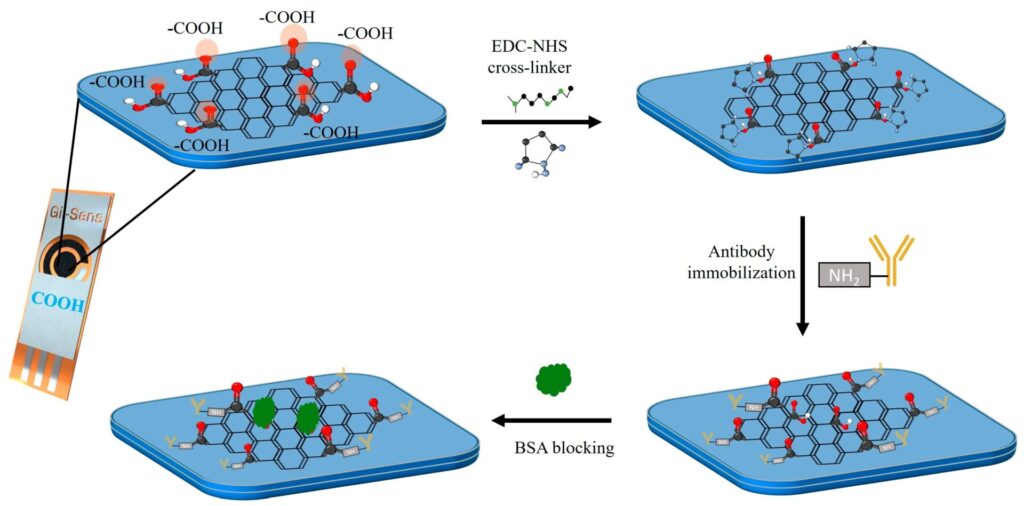
This study addresses this challenge by developing an electrochemical immunosensor using carboxyl-modified commercial graphene foam (COOH-GF) electrodes.
The conductivity of graphene is preserved by enabling efficient COOH modification through π–π non-covalent interactions, while antibody immobilisation is optimised via EDC-NHS carbodiimide chemistry.
The immunosensor detects tau-441, an AD biomarker, using differential pulse voltammetry (DPV), achieving a detection range of 1 fM–1 nM, with a limit of detection (LOD) of 0.14 fM both in PBS and human serum.
It demonstrates high selectivity against other AD-related proteins, including tau-217, tau-181, amyloid beta (Aβ1-40 and Aβ1-42), and 1% BSA. These findings underscore its potential as a highly sensitive, cost-effective tool for early AD diagnosis.
Introduction
Alzheimer’s disease (AD) is a severe neurodegenerative disorder characterised by progressive cognitive decline and functional impairment, ultimately leading to death [1]. It is the most common form of dementia, affecting millions of elderly individuals worldwide [2]. It is characterised by the formation of amyloid-beta (Aβ) plaques and neurofibrillary tangles made up of hyperphosphorylated tau proteins.
Tau is a microtubule-associated protein normally found in axons, where it stabilises the cytoskeletal structure. Under pathological conditions, tau becomes abnormally phosphorylated, leading to neuronal dysfunction and degeneration.
In AD patients, total tau and phosphorylated tau proteins have emerged as critical biomarkers for diagnosis and disease monitoring due to their strong association with disease progression [3,4].
Current methods for evaluating the phosphorylation of tau include enzyme-linked immunosorbent assays (ELISA) [5], polymerase chain reaction (PCR) [6], fluorescent immunosensors [7], surface plasmon resonance (SPR) [8,9], and piezoelectric biosensors [10].
However, these conventional techniques often lack the sensitivity needed to detect phosphorylated tau at early stages of disease. For example, while ELISA is effective for detecting tau proteins, it is insufficiently sensitive for identifying early-stage disease, resulting in diagnostic blind spots during critical windows.
Furthermore, tau phosphorylation is not unique to AD disease [11], it also occurs in other neurodegenerative disorders, reducing its diagnostic specificity as a diagnostic marker. These limitations underscore the need for advanced technologies capable of detecting AD with high sensitivity and specificity.
To date, various advanced methods have been developed for tau protein detection, including chemiluminescence ELISA [12], electrochemiluminescence [13], and nanopores [14]. However, these techniques face drawbacks including complex instrument maintenance, unstable luminescent reagents, and susceptibility to interference.
Electrochemical immunosensing technology, distinguished by its rapid response, high sensitivity, and miniaturisation potential, has emerged as a superior alternative [15,16]. Novel electrochemical detection methods based on tau are paving the way for precise monitoring.
Nazir, S.; Dogan, M.; Wei, Y.; Pan, G. Electrochemical Immunosensor Using COOH-Functionalized 3D Graphene Electrodes for Sensitive Detection of Tau-441 Protein. Biosensors 2025, 15, 465. https://doi.org/10.3390/bios15070465