Gii-based interdigitated microband arrays: [Fe(CN)6]3-/4- generator-collector redox processes and prussian blue catalyst attachment
Highlights
- Gii based interdigitated electrodes are robust and practical for voltammetry and generator-collector experiments
- Formation of Prussian blue triggered by positive applied potentials at the Gii-based electrodes to be deposited selectively and used as a collector electrode for the detection of hydrogen peroxide.
Abstract
Gii-based interdigitated microband array electrodes with typically 400 μm width and 150 μm inter-electrode gap (10 anodes and 10 cathodes, each 5.8 mm long; active array area 63.5 mm2) were produced on polyimide substrates and investigated for electrochemical performance and as a solution for the detection of hydrogen peroxide.
The one-electron reversible aqueous Fe(CN)63-/4- redox system is employed in the presence and in the absence of 0.1 M KNO3 supporting electrolyte.
Voltammetric responses on graphene foam suggest essentially reversible electron transfer under diffusion control with solution resistivity/migration contributing to the peak-to-peak separation especially in the absence of added electrolyte.
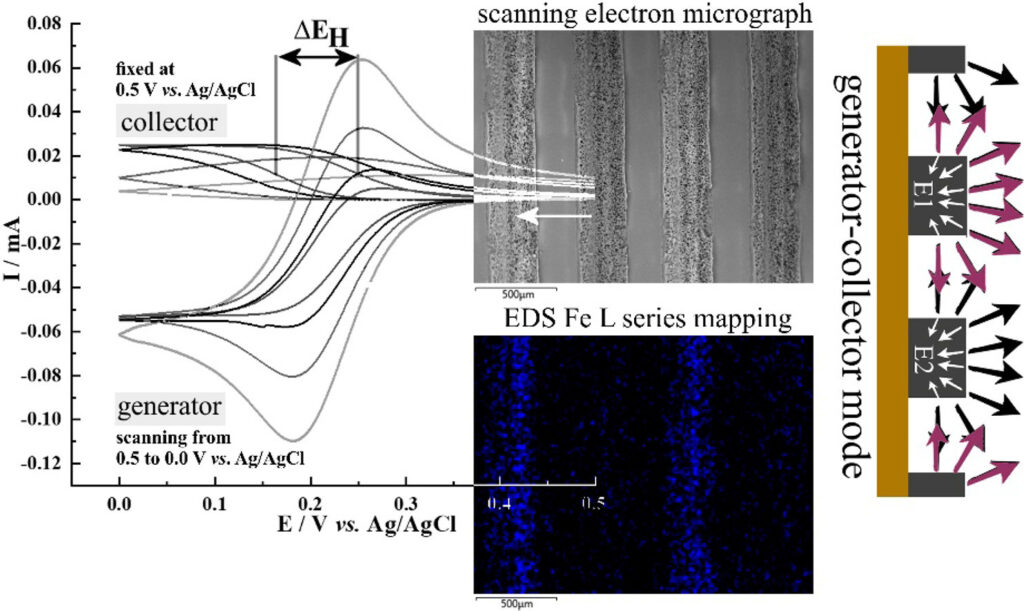
Under bipotentiostatic control, generator-collector feedback current signals are recorded with a signal hysteresis (affected by solution resistivity) consistent with a relatively wide inter-electrode gap.
With a positive potential bias during repetitive cyclic voltammetry, Prussian blue deposits/electrocatalysts form on the graphene foam. Generator-collector processes for combined oxygen reduction-hydrogen peroxide oxidation are observed.
Introduction
Interdigitated microband arrays have found widespread use in sensors [20,21] and in electrochemical generator-collector devices. Mixed oil|water redox systems have been investigated on interdigitated microband arrays.
Recently, interdigitated microband array electrodes have been proposed for electroorganic transformations in particular under paired reaction conditions (with a close anode-cathode gap to allow inter-electrode diffusion in a millisecond time domain) and without the need for added supporting electrolyte.
Interdigitated microband electrodes have been prepared for other types of electrode materials (Au; Pt; transparent ceramic films like ITO; boron-doped diamond; pyrolytic carbon) and they have been noted for laser-scribed graphene foam electrodes.
There could be benefits of interdigitated microband arrays based on porous materials especially in biosensing. Therefore, work on new types of graphene foam-based interdigitated microband array electrodes is required.
Tingran Liu, James E. Taylor, Pablo Lozano-Sanchez, Calum Doig, Joanne Holmes, Marco Caffio, Philip J. Fletcher, Frank Marken, Graphene foam interdigitated microband arrays: [Fe(CN)6]3-/4- generator-collector redox processes and prussian blue catalyst attachment, Electrochimica Acta, Volume 541, 2025, 147396, ISSN 0013-4686, https://doi.org/10.1016/j.electacta.2025.147396.